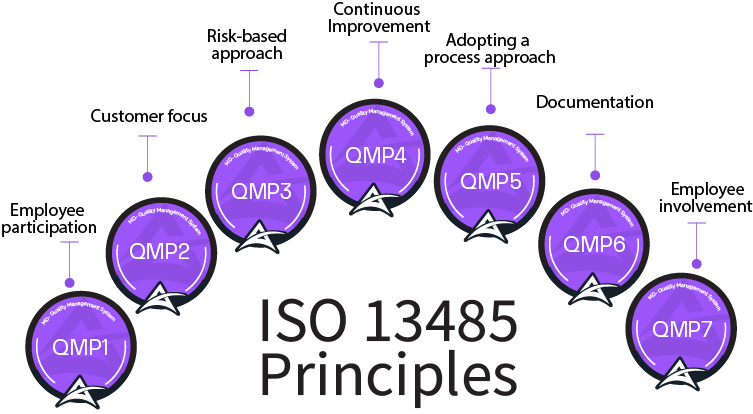
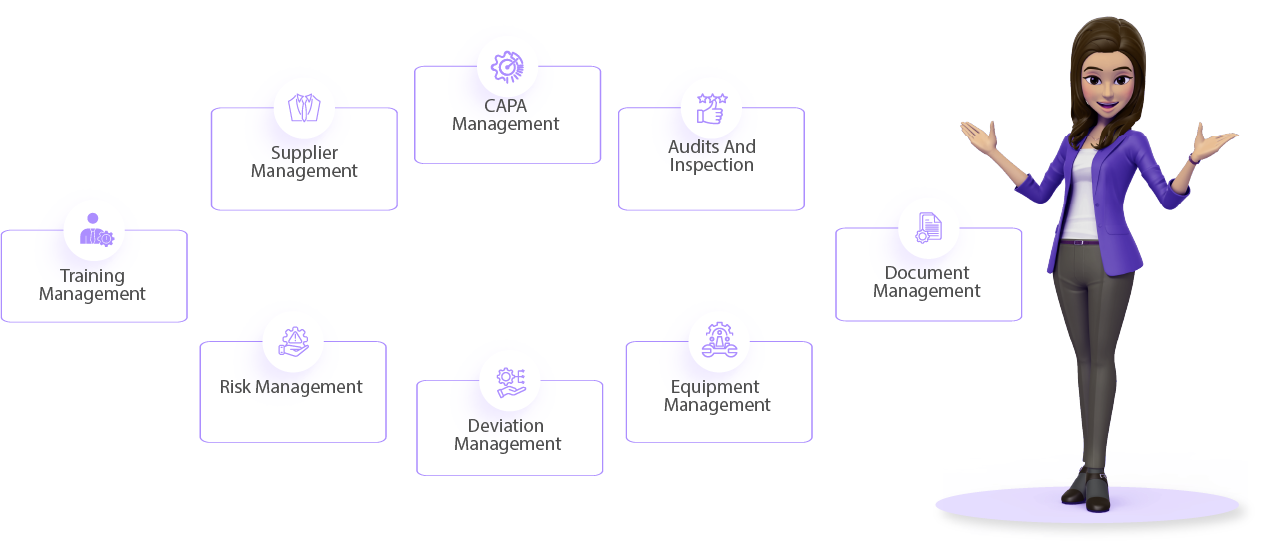
Axipro is a Business firm with record of winning many projects under tough circumstances.
Get clear, actionable solutions when you work with our industry-leading team of
exceptional and resourceful professionals.
Office 2181, Building 2648.
Road 5720, Block 257, The Lagoon, Amwaj Islands,
Kingdom of Bahrain
+973 3220 9587
Give us a call
info@axipro.co
24/7 online support